- Home »
- Applications »
- Single Molecule Applications
Single Molecule Applications
A single molecule experiment investigates the properties of individual molecules. More recently, single-molecule fluorescence is the subject of intense interest for biological imaging, to study single, labelled biomolecules in vivo in cells [39].
The photobleaching rates of 13 fluorescent dyes (see below), 4 of them conjugated to Trolox, were investigated in single-molecule measurements at 37oC in living cells and published in Nat. Chem. Biol. For this purpose the dyes were linked to a tag protein that was then fused to CD47.
In this study SeTau-647 (K9-4149 and K9-4148), a squaraine rotaxane dye, exhibited the best photobleaching performance with an exponential lifetime of 105.8 sec and the best localization precision with 21.5 nm. As a membrane-impermeable dye it allowed for the observation of up to 12,000 frames, which is the longest single-fluorescent-molecule tracking ever reported (see images below and paper in Nature Chem. Biol. [32]).
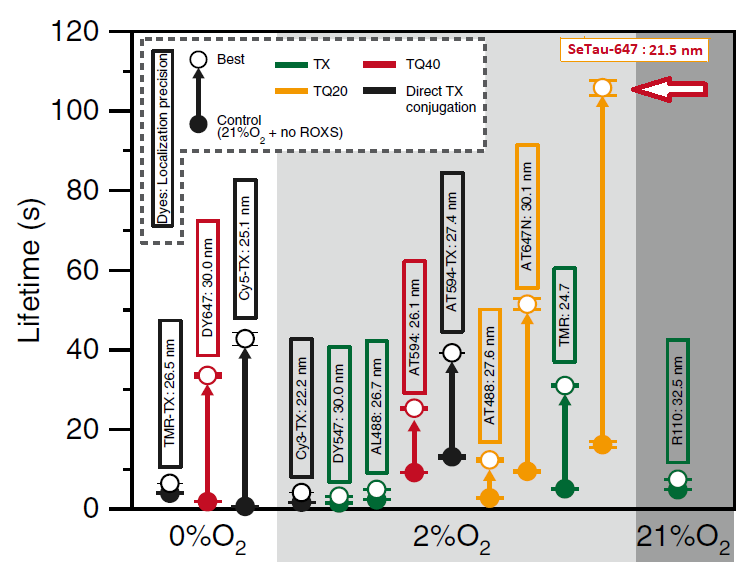
and Cy5-Tx) under controlled conditions and conditions for slowest photobleaching [32].
Most dyes including SeTau-647 (arrow) exhibited the longest photobleaching time at 2%O2.
SeTau-647 exhibited the best photobleaching performance with an exponential lifetime
of 105.8 sec and the best localization precision with 21.5 nm.
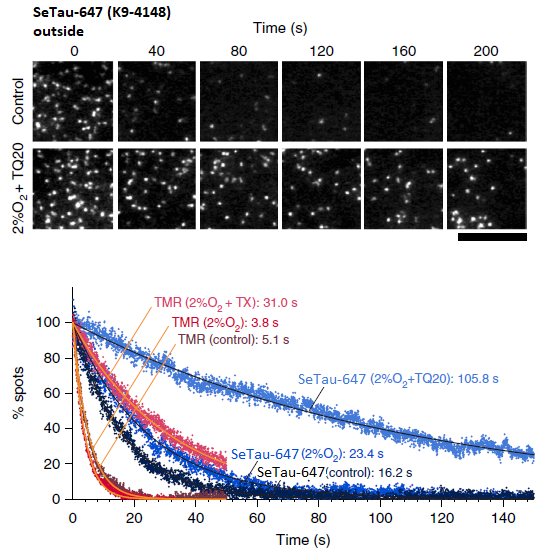
(fluorophore is located on the outer surface of a T24 epithelial cell).
Time-dependent reductions of the numbers of fluorescent spots found in each 33-ms
frame for TMR and SeTau-647 (K9-4148) on the extracellular surface (bottom) [32].
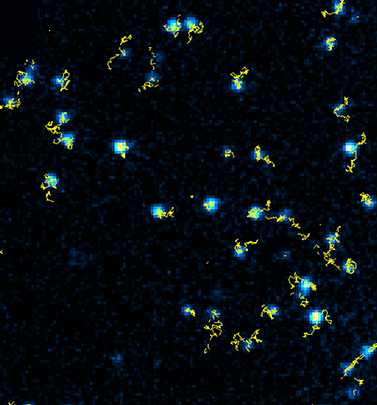
Single-molecule tracking with SeTau-647 (K9-4149)-labeled MEM-6/1 antibodies: the receptor CD-147 on the basal plasma membrane of human brain microvascular endothelial cells was followed for a duration of 100 s with a time resolution of 20 ms using TIRF microscopy and revealed the modulation of the CD-147 receptor mobility upon interaction [34].
The photophysical properties of SeTau-665 (K9-4119) were investigated on a plasmonic platform of self-assembled colloidal structures (SACS) of silver prepared on a semitransparent silver film and a SeTau-665-based immunoassay was performed on this platform and a control glass slide.
The fluorescence properties of SeTau-665 substantially change due to plasmonic interactions. While the average brightness increase of SeTau 665 in ensemble measurements was about 70-fold, fluorescence enhancements up to 400-times were observed on certain “hot spots” for single molecule measurements. The intensity increase is strongly correlated with a simultaneous decrease in fluorescence lifetime in these “hot spots”. The high increase in brightness allowed to reduce the excitation power resulting a reduced background and increased photostability.
on a glass and on an SACS surface. Top panels show photographs taken with 635 nm
excitation and observed through a 695 nm long-pass filter [16].
The remarkable fluorescence enhancement observed when using squaraine rotaxanes such as SeTau 665 on plasmonic platforms should allow not only reducing the detection limits in sensing devices but also enable single molecule measurements which were previously impossible.
| Excitation Light Sources | Characteristics | ||||||||||||||||||||
| Product Number (Specs Sheet) |
Product Name (Product Info) |
Target Group | 380 | 405 | 436 | 488 | 532 | 594 | 635 | 650 | 680 | 700 | 750 | 780 | Medium | λ abs [nm] |
ε [M –1 cm–1] |
λ em [nm] |
QY [%] |
FLT [ns] |
Buy |
| K8-1342 | Seta-670-NHS | NH2 | • | • | • | • | PB 7.4 | 667 | 180,000 | 688 | 7 | 0.42 | |||||||||
| K8-3335 | Seta-555-NHS | NH2 | • | PB 7.4 | 555 | 155,000 | 570 | 7 | |||||||||||||
| K8-3345 | Seta-555-DBCO | N3 | • | PB 7.4 | 555 | 155,000 | 570 | 7 | |||||||||||||
| K8-3346 | Seta-555-Azide | triple-CC | • | PB 7.4 | 555 | 155,000 | 570 | 7 | |||||||||||||
| K8-5035 | Seta-650-NHS | NH2 | • | • | PB 7.4 | 651 | 200,000 | 671 | 28 | ||||||||||||
| K8-5036 | Seta-650-Maleimide | SH | • | • | PB 7.4 | 652 | 200,000 | 672 | 28 | ||||||||||||
| K8-5045 | Seta-650-DBCO | N3 | • | • | PB 7.4 | 653 | 200,000 | 674 | 28 | ||||||||||||
| K8-5046 | Seta-650-Azide | triple-CC | • | • | PB 7.4 | 651 | 200,000 | 671 | 28 | ||||||||||||
| K9-4119 | SeTau-665-NHS | NH2 | • | • | • | PB 7.4 | 664 | 160,000 | 712 | 53 | 3.1 | ||||||||||
| K9-4142 | SeTau-647-di-NHS | NH2 | • | • | PB 7.4 | 650 | 200,000 | 694 | 65 | 3.2 | |||||||||||
| K9-4145 | SeTau-633-Ethyl-Ester | • | • | CHCl3 | 634 | 105,000 | 683 | 68 | |||||||||||||
| K9-4148 | SeTau-647-Maleimide | SH | • | • | PB 7.4 | 648 | 200,000 | 692 | 45 | 3.2 | |||||||||||
| K9-4149 | SeTau-647-NHS | NH2 | • | • | PB 7.4 | 649 | 200,000 | 695 | 61 | 3.2 | |||||||||||
| K9-4150 | SeTau-647 | • | • | PB 7.4 | 647 | 211,000 | 693 | 59 | 3.1 | ||||||||||||
| K9-4169 | SeTau-670-NHS | NH2 | • | • | • | PB 7.4 | 673 | 275,000 | 694 | 36 | 1.6 | ||||||||||
| K8-1341 | Seta-670-Maleimide | SH | • | • | • | • | PB 7.4 | 667 | 180,000 | 688 | 7 | ||||||||||
| K8-7522 | Seta-750-NHS | NH2 | • | • | • | PB 7.4 | 753 | 230,000 | 780 | 14 | |||||||||||
| K9-4179 | SeTau-680-NHS | NH2 | • | • | • | PB 7.4 | 683 | 295,000 | 705 | 58 | 2.9 | ||||||||||
| K9-3152 | SeTau-488-NHS | NH2 | • | PBS 7.4 | 486 | 59,000 | 532 | 27 | |||||||||||||
| K9-4159 new | SeTau-660-NHS | NH2 | • | PB 7.4 | 663 | 240,000 | 694 | 50 | 3.36 | ||||||||||||
Single Molecule (Homo)-FRET
Some of our Seta dyes e.g. Seta-670-NHS show extremely low blinking effects at the single molecule level and high photostability [16]. These labels exhibit easy conjugation chemistry and are available in both NHS and maleimide forms. Below is an example of a single molecule application that was done with Seta-670-NHS and a commercially available antibody.
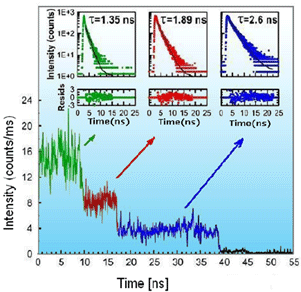
dye-to-protein ratio of 3. Insets to trajectories show values of fluorescence decay times for
the specific label [8].
Seta-670-NHS has a significant overlap integral between its absorption and emission spectra. Its Förster distance (R0) for homo-FRET was calculated to be about 50 A, a distance comparable to the size of the antibody. The homo-FRET is expected to occur already when an antibody is labeled with only two fluorophores and increases quickly with the number of labels. Analysis of single molecule traces shows an interesting behavior where efficient non-radiative excitation energy transfer and self-quenching already manifests itself with only 2 or 3 labels. Detailed analyses of the overall and average residence times reveal that multiple labeling with fluorophores, such as Seta-670-NHS, could be a good approach for increasing the number of available photons and extending the overall observation time to study/observe binding at the single antibody level. Intrinsically, the signal and lifetime of the individual, intermediate fluorescently labeled species strongly depend on the number of labels, but the average residence time for each single species is similar. Apparently due to energy transfer the change in fluorescence intensity is compensated by the change in average fluorescence lifetime. Contrary to ensemble measurements where over-labeling is commonly recognized as a problem, this approach appears to have significant advantages for single antibody (protein) studies [8, 17].
Fluorescence resonance energy transfer (FRET) is used to obtain distance information from 10 to 100 Ǻ, a range suitable for studying the global structure and interactions of biomolecules. Nevertheless, some conformational changes are difficult to detect using ensemble FRET. The development of single-molecule spectroscopy makes it possible to probe the conformational dynamics and interactions of biological systems at the single-molecule level.
To date the most popular fluorescent reporter molecules for single molecule studies are still small size organic molecules. Among them SeTau-647 is currently the dye with the longest exponential lifetime of 105.8 sec. Besides small size and brightness an ideal fluorescent reporter for single-molecule studies should exhibit good photostability and a low aggregation tendency including easy conjugation chemistry. FRET pairs should also have an adequate shift between the donor and acceptor emissions and similar brightness [17].
For additional FRET pairs we refer you to FRET applications, where we provide a more comprehensive list of donors and acceptors including the calculated Förster distances.

